Press Release

Metacen Therapeutics demonstrates the effectiveness of glycotoxin-degrading probiotic strains in alleviating fatty liver, hepatitis, and liver fibrosis
2024.09.26 17:51- 작성자 관리자
- 조회 2,764
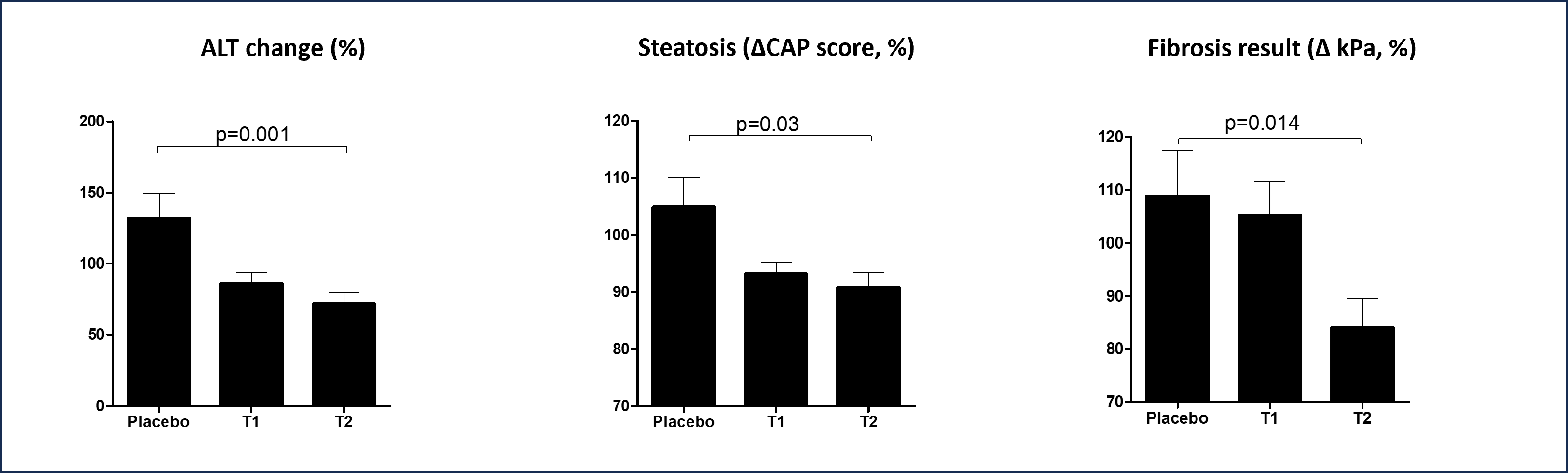
MetaCen Therapeutics (CEO Myoung-Gyu Park) announced that they have confirmed significant effects of MCT192, a proprietary compound containing glycotoxin-degrading probiotic strain KF140, in a human clinical trial involving 90 adults with early symptoms of metabolic dysfunction-associated fatty liver disease (MAFLD), such as diabetic fatty liver, hepatitis, and liver fibrosis.
The clinical trial, conducted over approximately two years in collaboration with Bundang Seoul National University Hospital and Yongin Severance Hospital, evaluated the efficacy and safety of the compound MCT192 in patients with mild fatty liver, hepatitis, and fibrosis, including those with diabetes. Statistically significant differences were observed between the control group and the test group in key markers such as ALT, AST, and γ-GTP, meeting safety evaluation criteria as well. The study also showed significant results in other markers like HbA1c, fibrosis score (kPa), liver fibrosis (APRI), steatosis score (CAP), and HIS (Hepatic Steatosis Index), indicating potential benefits for patients with diabetic fatty liver and liver fibrosis, key conditions associated with MAFLD.
The test substance, MCT192, used in this trial is based on ingredients that lower glycotoxin levels, including glycotoxin-reducing probiotics, amino acids, and fat-soluble antioxidants. Glycotoxins are harmful compounds formed by the chemical reaction between reducing sugars like glucose and fructose and amine groups in proteins, fats, and DNA. These substances are produced during cooking, within the body, and by gut bacteria. Also known as Advanced Glycation End-products (AGEs), they are metabolic by-products formed when carbohydrates and proteins are not adequately metabolized and transported through blood vessels to cells. Accumulation of glycotoxins in the blood or tissues can cause systemic disturbances, triggering excessive inflammatory responses and leading to chronic diseases such as cardiovascular disease, diabetes, and cancer. AGEs are also considered a marker of biological aging and are influenced significantly by diet and lifestyle.
Fatty liver, a common condition among modern people, occurs when excessive fat accumulates in the liver. Steatohepatitis, on the other hand, is characterized by fat accumulation along with inflammation and necrosis of liver cells. Poor dietary habits, such as high-calorie, high-fat, and high-sugar diets, combined with a lack of exercise, are the primary causes. Glycotoxins and lipid toxins, which contribute to inflammation and liver damage, are the main factors driving the progression from fatty liver to steatohepatitis and fibrosis. As there are currently no officially recognized treatments for fatty liver, exercise and diet are the most crucial therapeutic methods. Therefore, the results of this clinical trial are promising for the treatment of fatty liver.
Myoung-Gyu Park, CEO of MetaCen Therapeutics, stated, “This clinical trial has confirmed that MCT192 is safe for human consumption and has potential benefits for improving liver function. Based on these findings, we plan to conduct follow-up studies and additional clinical trials to develop this compound as a new drug. The results are promising for our approach to NASH/MAFLD, a field currently lacking reliable preventive and therapeutic agents, and are also highly significant from a business perspective. We hope that this breakthrough will offer new hope to the many patients suffering from MAFLD, one of the most prevalent chronic diseases related to metabolic syndrome."
Founded in 2015, MetaCen Therapeutics is a specialized healthcare R&D company that has built a broad pipeline based on glycotoxin-reducing technology, focusing on nutraceuticals and microbiome-based pharmaceuticals. As a leader in glycotoxin-related healthcare, the company has developed a variety of nutraceuticals and health supplements using its proprietary five core technologies, which are sold through over 1,000 member pharmacies nationwide.
Reporter: Dae-Sung Kim kdsung@dt.co.kr
Source: [Digital Times] (https://www.dt.co.kr)
